Table of Contents Show
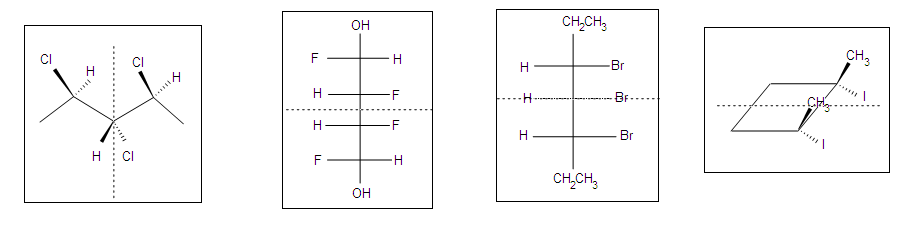
Meso Compound – Meso Compound is a compound with two chirality centers where there is the same set of four groups at each chirality center, the combination where the four groups are arranged such that the centers are mirror images of each other (i.e. where the molecule has an internal mirror plane). For example consider 2,3-dichlorobutane. There are two chirality centers (C2 and C3), each with -H, -Cl, -CH3 and the other -CHClCH3 groups attached. Since there are two chirality centers, then, at least in principle, there are 4 possible permutations of the stereocenters : (R,R), (R,S), (S,R) and (S,S)
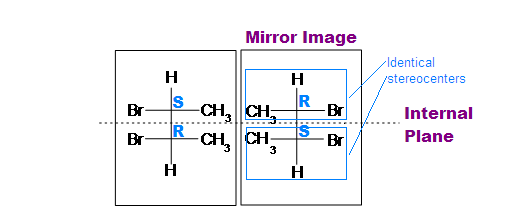
The (R,R) and (S,S) structures are non-superimposable mirror images, so they are a pair of enantiomers.
If we look at the (R,S) and (S,R) as drawn above, it should be reasonably easy to recognise that these two structures are actually the same thing because they are superimposable, i.e. (R,S) º (S,R). This structure is the meso isomer. To verify this, rotate one of the models 180 degrees about the vertical axis to make it look the same as the other structure. In the conformations of the (R,S) shown above, the mirror plane should be obvious (vertical plane bisecting the middle of the central C-C bond).
However, let’s look at the other important conformation of the meso isomer and make sure we can recognise it is the meso isomer.
The relationship of A to B is not immediately obvious. However, once the right hand end has been rotated about the central C-C bond by 180 degrees, A can been seen to be the mirror image of B . A quick way of recognising whether a molecule is achiral is to look for a plane of symmetry.
Meso Compound Identification
If A is a meso compound, it should have two or more stereocenters, an internal plane, and the stereochemistry should be R and S.
- Look for an internal plane, or internal mirror, that lies in between the compound.
- The stereochemistry (e.g. R or S) is very crucial in determining whether it is a meso compound or not. As mentioned above, a meso compound is optically inactive, so their stereochemistry should cancel out. For instance, R cancels S out in a meso compound with two stereocenters.
.bmp?revision=1&size=bestfit&width=375&height=171)
trans-1,2-dichloro-1,2-ethanediol
.bmp?revision=1&size=bestfit&width=433&height=183)
(meso)-2,3-dibromobutane
Tips: An interesting thing about single bonds or sp3-orbitals is that we can rotate the substituted groups that attached to a stereocenter around to recognize the internal plane. As the molecule is rotated, its stereochemistry does not change. For example:
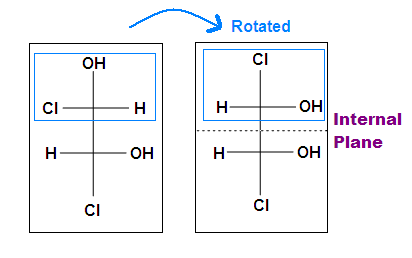.bmp?revision=1&size=bestfit&width=412&height=258)
Another case is when we rotate the whole molecule by 180 degree. Both molecules below are still meso.
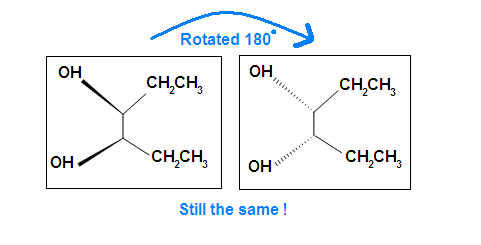
Remember the internal plane here is depicted on two dimensions. However, in reality, it is three dimensions, so be aware of it when we identify the internal mirror.
Example
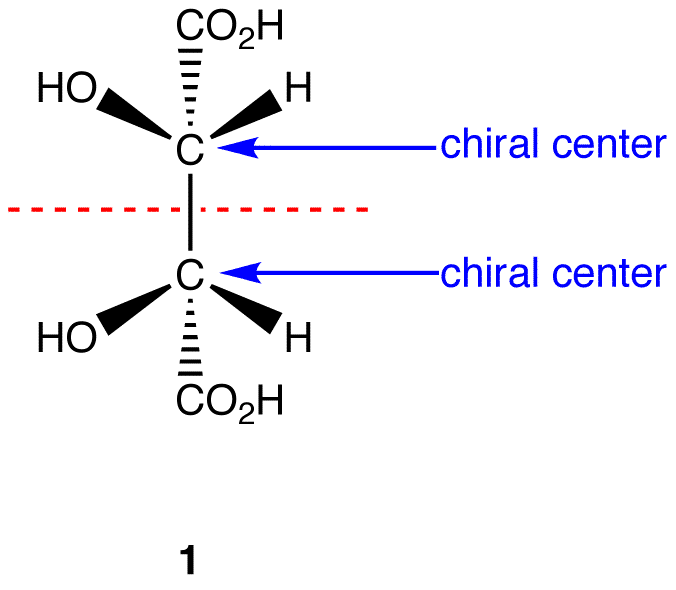
This molecule has a plane of symmetry (the horizontal plane going through the red broken line) and, therefore, is achiral; However, it has two chiral carbons and is consequentially a meso compound.
Other Examples of meso compounds
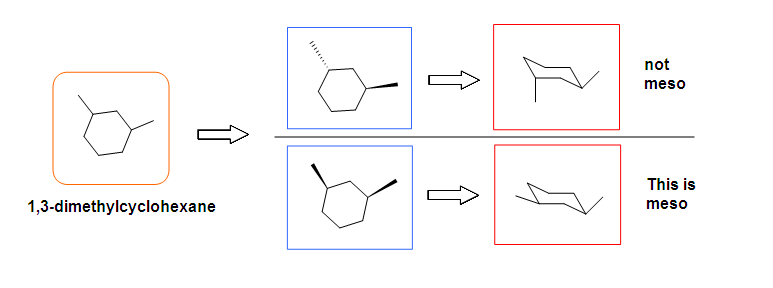
Meso compounds can exist in many different forms such as pentane, butane, heptane, and even cyclobutane. They do not necessarily have to be two stereocenters, but can have more.
.bmp?revision=1&size=bestfit&width=695&height=176)
Optical Activity Analysis | Meso Compound
When the optical activity of a meso compound is attempted to be determined with a polarimeter, the indicator will not show (+) or (-). It simply means there is no certain direction of rotation of the polarized light, neither levorotatory (-) and dexorotatory (+).
Achiral Diastereomers (meso-Compounds)
The chiral centers in the preceding examples have all been different. In the case of 2,3-dihydroxybutanedioic acid, known as tartaric acid, the two chiral centers have the same four substituents and are equivalent. As a result, two of the four possible stereoisomers of this compound are identical due to a plane of symmetry, so there are only three stereoisomeric tartaric acids. Two of these stereoisomers are enantiomers and the third is an achiral diastereomer, called a meso compound. Meso compounds are achiral (optically inactive) diastereomers of chiral stereoisomers. Investigations of isomeric tartaric acid salts, carried out by Louis Pasteur in the mid 19th century, were instrumental in elucidating some of the subtleties of stereochemistry. Some physical properties of the isomers of tartaric acid are given in the following table.
| (+)-tartaric acid: | [α]D = +13º | m.p. 172 ºC |
| (–)-tartaric acid: | [α]D = –13º | m.p. 172 ºC |
| meso-tartaric acid: | [α]D = 0º | m.p. 140 ºC |
Fischer projection formulas provide a helpful view of the configurational relationships within the structures of these isomers. In the following illustration a mirror line is drawn between formulas that have a mirror-image relationship. In demonstrating the identity of the two meso-compound formulas, remember that a Fischer projection formula may be rotated 180º in the plane.

Problems | Meso Compound
Beside meso, there are also other types of molecules: enantiomer, diastereomer, and identical. Determine if the following molecules are meso.
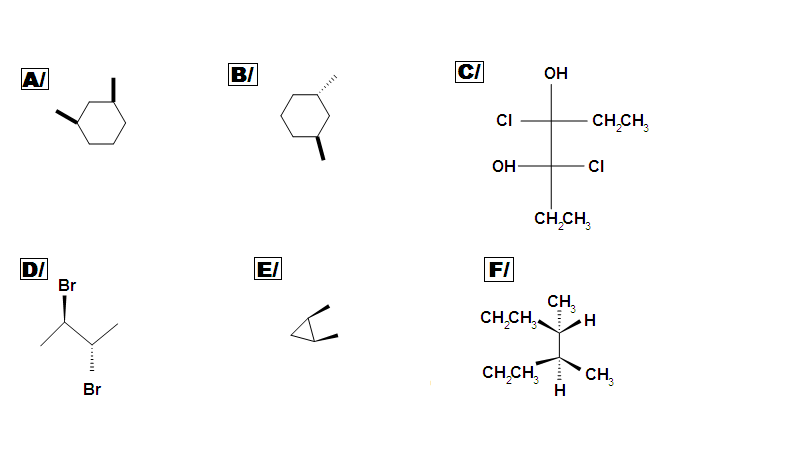
Answer key: A C, D, E are meso compounds.


